OEM and Private Label Opportunities: A Guide for Diagnostic Brand Owners
SEO Keywords: OEM magnetic beads, private label IVD reagents, bulk silica beads, biotech supply chain, customized reagent manufacturing, diagnostic brand scaling.
Introduction: Accelerating Market Entry in the IVD Space
The global In Vitro Diagnostics (IVD) market is more competitive than ever. For companies looking to launch a new DNA/RNA extraction kit, the "Time-to-Market" is a critical competitive advantage. Building a manufacturing facility for silica magnetic beads from scratch is a multi-million dollar, multi-year endeavor. This is why savvy diagnostic brand owners and CEOs are turning to OEM (Original Equipment Manufacturer) and Private Label partnerships.
The Strategic Value of OEM Sourcing
Choosing an OEM partner for silica magnetic beads allows a company to focus on its core strength: assay design and clinical validation.
-
Capital Expenditure (CAPEX) Reduction: By outsourcing bead synthesis, firms avoid the high costs of chemical reactors, cleanroom maintenance, and specialized chemical engineering staff.
-
Access to Proprietary Technology: Top-tier OEM providers offer beads with advanced silica-coating techniques that provide superior purity and "non-leaching" properties, which might be difficult to develop in-house.
Customization: Beyond the "Off-the-Shelf" Solution
A true OEM partnership is not just about buying a generic product; it’s about customization. Engineers can request specific modifications to fit their unique instrument or protocol:
-
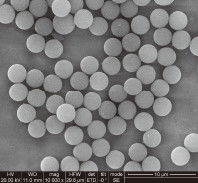
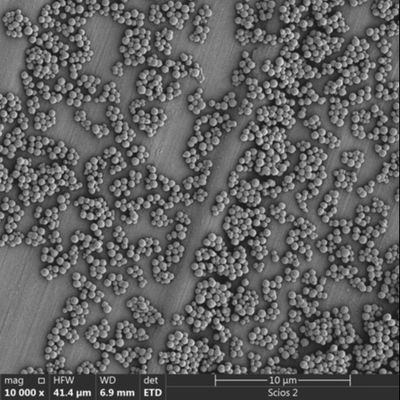
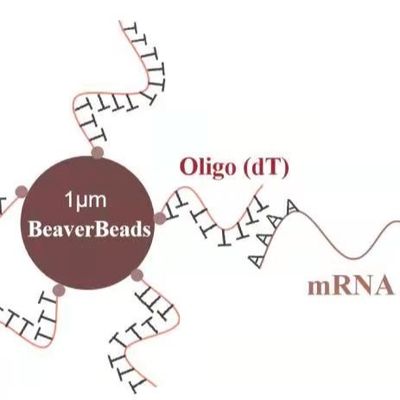
Particle Size Customization: Whether your system requires $0.5 mu m$ beads for maximum surface area or $3.0 mu m$ beads for rapid settling, OEM providers can tune the synthesis.
-
Buffer Compatibility: OEM beads can be supplied pre-suspended in the client's proprietary lysis or binding buffers, simplifying the final packaging process.
-
Concentration Tuning: Adjusting the "mg/mL" concentration to match the pipetting requirements of specific robotic platforms like the Hamilton Microlab STAR or Tecan Freedom EVO.
Quality Systems and Regulatory Support
For the procurement and legal teams, the "Private Label" model must be backed by a robust quality management system.
-
White-Label Documentation: A high-quality OEM partner provides a full "Technical File," including stability data, Certificates of Analysis (CoA), and Material Safety Data Sheets (MSDS) that the brand owner can use for their own regulatory filings (e.g., FDA 510(k) or IVDR).
-
Batch Reservation: To ensure supply chain security, OEM agreements often include "Batch Reservation" clauses, guaranteeing that the brand owner has access to the same lot of beads for 12-24 months.
The Path to Scaling: From Pilot to Bulk Production
Most OEM journeys start with a small pilot phase (e.g., 100mL of beads) for R&D validation. As the product gains market traction, the supplier must be able to scale to 10L, 50L, or even 100L batches without a loss in performance. This scalability is the hallmark of a world-class silica magnetic bead manufacturer.
Conclusion: Building a Lean Diagnostic Brand
In 2026, the leanest and fastest companies will win. By leveraging OEM silica magnetic beads, diagnostic brands can maintain high quality and low overhead, allowing them to pivot quickly to new diagnostic challenges.

 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!