Troubleshooting Common Issues: Yield, Purity, and Bead Carryover in Automated Labs
SEO Keywords: Troubleshooting magnetic beads, DNA yield optimization, magnetic bead carryover, PCR inhibition, automated extraction problems, silica bead aggregation.
The High Stakes of Automated Extraction
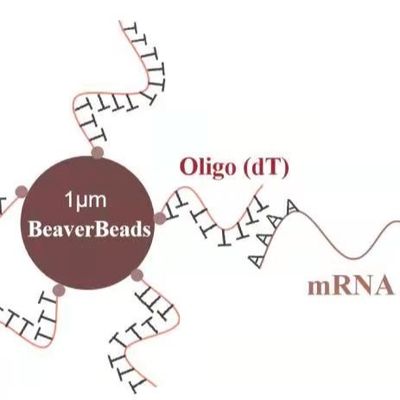
In a high-throughput diagnostic environment, a 5% failure rate isn't just a technical nuisance—it’s a massive financial drain and a potential risk to patient outcomes. When automated nucleic acid extraction protocols fail, the culprit is often found at the interface of the silica magnetic bead and the sample matrix. For laboratory managers and automation engineers, identifying the root cause of low yield or poor purity is essential for maintaining operational efficiency.
Problem 1: Suboptimal DNA/RNA Yield
Low yield is the most common complaint in molecular diagnostics. From an engineering perspective, this usually stems from a failure in the "Binding" phase.
-
Chaotropic Salt Concentration: Silica binding is dependent on the dehydration of the DNA backbone. If the lysis buffer is diluted by the sample (e.g., a larger-than-expected urine or plasma volume), the salt concentration may drop below the threshold required for efficient binding.
-
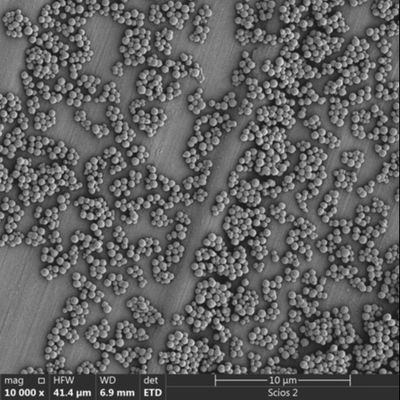
Bead-to-Sample Ratio: More beads do not always mean more DNA. Excessive bead concentrations can lead to "crowding," where the beads shield each other from the target molecules.
-
Incubation Dynamics: In automated systems, the mixing speed must be high enough to keep beads in suspension but gentle enough to avoid shearing long-genomic DNA.
Problem 2: Poor Purity and Salt Carryover
High yield is useless if the resulting sample is "dirty." The presence of residual salts (Guanidine) or proteins can inhibit downstream PCR or NGS library prep.
-
The "Washing" Bottleneck: Most impurities are trapped within the "bead clump" during magnetic separation. Engineers should optimize the "off-magnet" resuspension time during wash steps to ensure the beads are fully dispersed and the impurities are released into the wash buffer.
-
Ethanol Carryover: If the drying step is too short, residual ethanol remains on the silica surface. Ethanol is a potent PCR inhibitor. Conversely, over-drying the beads can make them "crack," trapping the DNA and making elution nearly impossible.
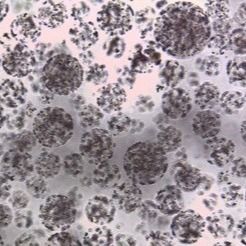
Problem 3: The "Silent Killer"—Bead Carryover
Bead carryover occurs when small amounts of magnetic particles are aspirated along with the eluate.
-
Interference with Optics: In real-time PCR, residual iron oxide particles can absorb or scatter light, leading to "noisy" baseline readings or false negatives.
-
Enzymatic Inhibition: While silica is inert, the iron core of the bead can leached if the elution buffer's pH is too low or if the beads are left in the eluate for extended periods.
-
Solution: Implementing a "Double Magnet" step—where the eluate is moved to a fresh plate and placed on a magnet a second time—is a best practice in automated liquid handling.
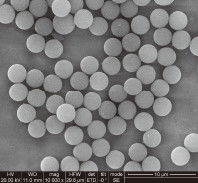
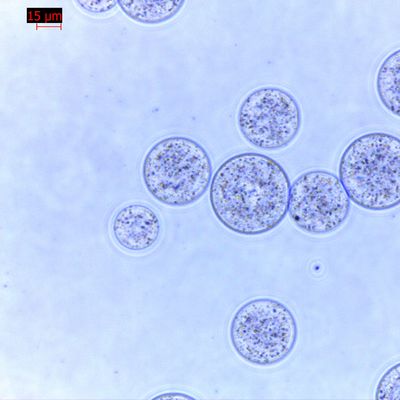
Problem 4: Bead Aggregation and "Clumping"
If beads do not resuspend easily, the automation software will struggle to deliver accurate volumes.
-
Storage Temperature: Freezing silica magnetic beads can permanently damage the silica shell and lead to irreversible aggregation.
-
pH Shifts: If the storage buffer shifts toward an acidic pH, the surface charge of the silica approaches its isoelectric point, causing the beads to stick together.
Conclusion: Data-Driven Optimization
For B2B clients, the solution to these problems lies in standardization. By sourcing high-quality silica beads with a certified narrow size distribution and using "Open-System" compatible protocols, labs can minimize downtime and maximize the value of their diagnostic assets.

 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!